
Biopharmaceutical
Dezyme offers a valuable solution for the development of new biologics or processes. Biopharmaceutical companies can use Dezyme software to rapidly detect regions with stability weaknesses and identify relevant mutations.
Our software could contribute to:
- Receptor affinity and selectivity improvement
- Protein stabilization and solubilization
- Guarantee good bioavailability and biodistribution properties
Case Studies
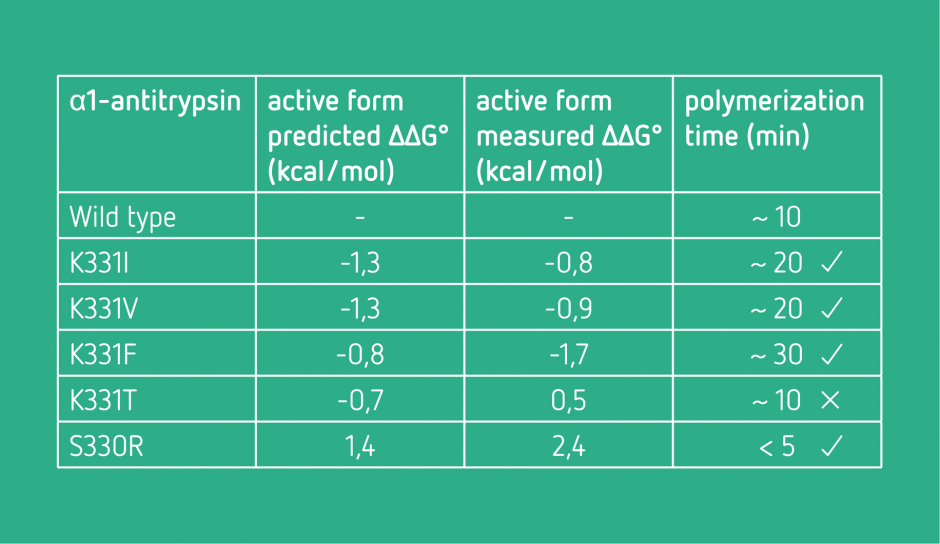
Conformational stabilization
The objective was to find mutations stabilizing the active form of a protein and destabilizing its polymerized form, which causes a range of disease.
Negative values suggest a stabilization. The polymerization time was used as indicator of the quality of identified mutations.
After the experimental validation, four of the five predictions were in agreement with the predictions.